Overview of SPMED™ Human Recombinant Enzymes
SPMED™ Human Recombinant Enzymes
SPMED™ is a product that can be widely used in new drug development and pharmaceutical and biomedical research based on research on substrate and inhibition characteristics.
We provide the high-quality enzymes recommended by the US Food and Drug Administration (FDA) for new drug development and drug interaction research, such as cytochrome P450 (CYPs) and UDP-glucuronosyltransferase (UGTs), which are expressed with a reductase in insect cells using a baculovirus expression system.
Features & Benefits
-
-
Substrate specificity similar to the native enzyme inside the human body

-
-
-
Production and verification using high expression system
: Provides high quality, high activity enzymes 
-
-
-
Customized production including additional metabolic enzymes and diverse variant enzymes

-
-
-
Increased time efficiency and economic efficiency by reducing delivery time through mass production in Korea
-
-
-
Reliability is guaranteed by providing performance test results on activity, stability, etc.

-
-
-
A package of 8 types of CYP recommended by the FDA CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A4, and CYP3A5)
-
Product Performance Test Items
-
Activity : Determination of activity level in relation to specific substrates
-
Stability during freezing and thawing : Measuring changes in activity while freezing and thawing the enzyme at least 6 times
-
Linearity with time: Excellent metabolite production efficiency of over 30 minutes
-
Provides varied information re. CYPs (specific P450 content, cytochrome b5 content, cytochrome c reductase activity)
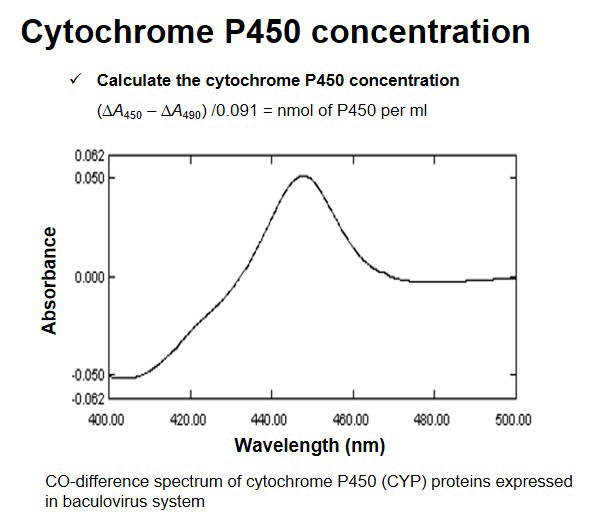
Product List
In addition to the following items, you can order and produce them according to the client's request, such as variable types and additional metabolic enzymes.
Drug reaction phenotyping metabase pool package for screening in research
FDA Recommended 8 Major CYP Types : CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP3A4, CYP3A5
Overall procedure for the production of drug metabolism enzymes (DMEs)
-
-
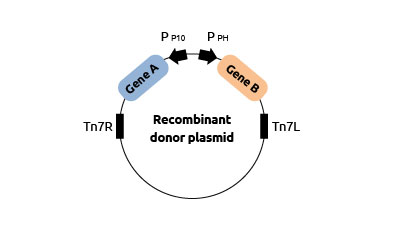
Generating
the recombinant donor plasmid
-
-
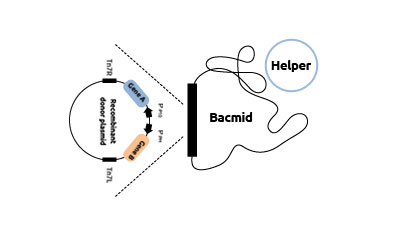
Transposition of donor plasmid
into a baculovirus shuttle vector (bacmid)
-
-
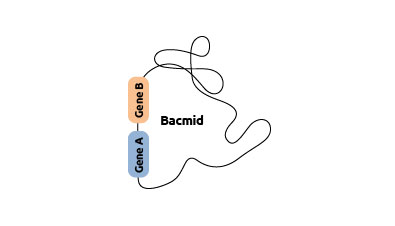
Isolating
the recombinant bacmid DNA
-
-
Transfection of insect cells
with recombinant bacmid DNA
-
-
Virus amplification and Expression
(Scale-up)
-
-
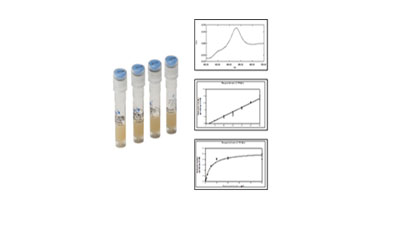
Recombinant protein extraction
& Activity / validation
